About Oxalic Acid
All products are for external use only
Free shipping notes: 40 Lb Bag in Box
40 Lb Bag in box ships free in lower 48 continental states. Soapgoods Inc reserves the right to ship free shipping items via any carrier.
Documentation
Identification
- Synonyms: Oxalic Acid, Ethanedioic acid, Oxalic acid (aqueous), Oxalic acid dihydrate
- INCI Name: Oxalic Acid
- CAS: 144-62-7
- Einecs: 205-634-3
The Science
- Grade: DiHydrate, Technical 99.6%
- Viscosity: Solid Powder
- Solublity: Soluble in water
- Storage: Cool, dark dry area, air tight container preferred
Characteristics
- Appearance: White granules.
- Odor: ??
- Natural: Occurs naturally in cabbage, spinach and rhubarb leaves.
- Packaging: 1 Lbs is a single bag, 5 Lb is a single bag, 55 lb is a single bag. 550 lb is 10 x 55 lb bags.
- Shelf life: Suggested retest date 2 year from purchase
Usage / Benefits
- Industries: Cleaning and Polishing, Textile, Leather, Pharmaceuticals, Electronics, Agriculture, Metallurgy, Mining, Photography, Cosmetics, Chemical manufacturing
- Applications: Cleaning Agent for Rust Removal, Bleaching Agent for Wood and Laundry, Industrial Manufacturing (including dyes, inks, and pharmaceuticals), Mordant in Dyeing Processes, Textile Purification and Bleaching, Ink Stain Removal, Wastewater Treatment, Leather Tanning and Finishing
- Benefits: Effective Rust Remover, Efficient Stain Remover (such as ink stains), Essential in Various Industrial Processes (including dyeing, leather treatment), Useful as a Bleaching Agent for Wood and Textiles, Important Component in Pharmaceutical Manufacturing, Versatile Cleaning Agent (for metals, ceramics, etc.), Plays a Role in Water Purification and Treatment
- Products Uses: Rust Removal in Various Surfaces, Cleaning Agent for Metals and Ceramics, Leather Tanning Process, Wood Bleaching and Restoration, Textile Bleaching, Mordant in Dyeing Processes, Wastewater Treatment Agent, Component in Pharmaceuticals, Stain Remover (such as ink stains), Precursor in Organic Synthesis, Raw Material in Various Industrial Chemical Reactions
- Safety: Generally safe, however keep out of eyes and do not eat.
- Cautions: Not for ingestion, keep away from pets and children who may attempt to eat.
- External Use Only: Even if food grade, we do not provide items for ingestion, all of our items are for external use only.
Oxalic Acid
It is a white solid used in removal of certain kinds of stains, in removing calcium ions from solutions, and in tanning leather. It occurs naturally and is toxic. The metabolism of sugar by many species of mold results in the production of oxalic acid. The most common uses of oxalic acid are in tanning leather and removing rust and ink stains. In stain removal, it acts as a reducing agent uses:
Oxalic acid is a naturally occurring compound found in many plants and vegetables. Chemically, it's represented as C2H2O4 and is known for its sharp, crystalline structure. While it has multiple applications across industries, its role in personal care, soap making, and cosmetic formulations is nuanced.
Chemical Properties
As a dicarboxylic acid, oxalic acid has two carboxyl groups, which makes it a relatively strong acid. This property can be harnessed in cosmetic formulations, especially when aiming to adjust the pH or chelate metal ions. The acid is generally available as a dihydrate in its crystalline form, but it's the anhydrous form that is predominantly used in industrial applications.
Role in Personal Care
Oxalic acid, while not a primary ingredient in many personal care products, can serve a supplementary role. It's occasionally used as a pH adjuster or buffering agent in certain formulations. Its strong acidic nature ensures that a minimal amount is required to alter the pH of a product, especially if the goal is to make the product more acidic.
Cosmetic Formulations
Within the realm of cosmetics, oxalic acid can play a unique role as a chelating agent. Chelating agents are compounds that can form several bonds with a single metal ion, making them particularly useful in cosmetic formulations to stabilize and enhance the performance of the product. By binding with metal ions, oxalic acid can prevent the degradation of the product and improve its shelf life.
However, due to its acidic nature and potential for skin irritation, its concentration levels in cosmetic products are typically kept low. It's crucial to strike a balance to harness the benefits of the acid while ensuring that the end product remains safe for consumer use.
Soap Making
In soap making, the primary role of acids is to react with bases (like lye) to produce salts, which in the case of soap making, results in the formation of the soap itself. However, the most commonly used acids in this process are fatty acids, which are found in oils and fats. Oxalic acid isn't a primary player in traditional soap making, but it might find occasional use in specialty soaps or to serve specific purposes like adjusting pH or enhancing clarity.
Challenges and Precautions
Despite its potential benefits, there are challenges and precautions to be considered when using oxalic acid in personal care and cosmetic formulations. One of the main concerns is the acid's potential to cause skin irritation, especially when used in higher concentrations. Thus, its use needs to be approached with caution, ensuring that the product remains safe for its intended use.
Furthermore, oxalic acid is known to form crystals readily. When these crystals form in a product, they can create a gritty texture that might not be desirable, especially in skin care or hair care formulations. As such, product developers need to ensure that the formulation remains stable and free from unwanted crystallization.
Oxalic Acid Uses
Oxalic acid, a simple dicarboxylic acid, boasts a range of applications across various industries, thanks to its unique chemical properties. Here are some of the noteworthy uses of oxalic acid:
Personal Care and Cosmetics
- pH Adjuster: Due to its acidic nature, oxalic acid can be used to adjust the pH of cosmetic and personal care products, ensuring they remain skin-friendly and effective.
- Chelating Agent: Oxalic acid can bind to metal ions, preventing them from reacting with other ingredients in a product. This is especially beneficial in cosmetics as it can help stabilize and enhance product performance, increasing shelf life.
- Clarifying Agent: In certain formulations, oxalic acid might be used to improve the clarity or transparency of a product, giving it a more polished appearance.
Cleaning and Rust Removal
- Stain Remover: Oxalic acid is effective in removing iron and rust stains. It's often found in specialty cleaning products designed for such purposes.
- Tile and Stone Cleaner: The acid can be used to clean tiles and stones, effectively removing grime and other stubborn stains.
- Bleaching Agent: In some instances, oxalic acid acts as a bleaching agent, restoring the color of wood and other surfaces.
Metal Treatment and Production
- Extraction Processes: Oxalic acid is sometimes used in metallurgy, aiding in the extraction of certain minerals from their ores.
- Metal Passivation: The acid can help form a protective layer on certain metals, preventing them from reacting with the environment and corroding.
Textile Industry
- Dyeing and Printing: Oxalic acid plays a role in the dyeing and printing processes of textiles, ensuring that colors bind well to the fabric.
- Stripping Agent: Used to strip away unwanted colors or residues, preparing the fabric for a fresh round of dyeing.
Other Uses
- Research & Laboratory: In scientific research, oxalic acid can be a reagent for various chemical reactions.
- Insecticide: Certain formulations utilize oxalic acid as a treatment against Varroa mites in beehives.
Oxalic Acid Benefits
Cleaning Agent
Oxalic acid is commonly used as a cleaning agent, particularly for rust removal and cleaning metals. It reacts with rust to form a soluble complex that can be easily rinsed away.
Bleaching Agent
It serves as a bleaching agent for both wood and laundry. In woodwork, it's used to lighten wood stains. In laundry, it can be used to remove tough stains and brighten fabrics.
Industrial Applications
In industries, oxalic acid is used in the manufacture of various products, including dyes, inks, and pharmaceuticals. It acts as a mordant in dyeing processes and is an essential intermediate in various chemical synthesis processes.
Textile Industry
Within the textile industry, oxalic acid is used to purify or bleach textiles and to remove ink stains.
Waste Water Treatment
Oxalic acid can be utilized in wastewater treatment as it helps in the removal of calcium and other unwanted minerals, contributing to the water purification process.
Leather Industry
In the leather industry, oxalic acid is used in tanning and finishing leather products. It helps in stabilizing the collagen or protein fibers in leather.
Health and Safety Considerations
It is important to note that oxalic acid can be toxic if ingested in large quantities, and it should be handled with care, following proper safety guidelines.
FAQ
Oxalic Acid as Wood Bleach
Oxalic acid is a potent compound frequently used in various industries for its bleaching and cleaning capabilities. One of its notable applications is in the domain of woodworking, where it serves as a wood bleach.
What is Oxalic Acid?
Oxalic acid is a colorless, crystalline organic compound that belongs to the family of dicarboxylic acids. When it comes to woodworking, its bleaching properties come into play, offering a solution to several wood discoloration problems.
Benefits of Using Oxalic Acid in Wood Bleaching
Applying oxalic acid on wood surfaces can result in multiple advantages.
- Stain Removal: Oxalic acid is effective in removing stubborn stains, particularly those caused by water, rust, or the natural aging of wood.
- Uniform Color: It can help even out color inconsistencies or dark spots, leading to a more uniform appearance.
- Preparation for Finishing: By evening out the wood tone, oxalic acid can make the surface more amenable to finishing processes like staining or varnishing.
Precautions with Oxalic Acid
Given its potent nature, there are certain precautions to observe when using oxalic acid on wood.
- Safety First: Always wear protective gloves and eyewear when handling oxalic acid. Ensure that the workspace is well-ventilated.
- Concentration: Dilute the oxalic acid appropriately as per the requirements of the task. Overconcentration can harm the wood.
- Neutralization: After treatment, it's essential to neutralize the acid by rinsing the wood with water or using a base like baking soda.
- Disposal: Ensure that any leftover solutions or residues are disposed of according to local regulations, as oxalic acid can be harmful to the environment.
Conclusion
Oxalic acid serves as a powerful wood bleach, addressing discoloration and preparing surfaces for further finishing. However, its potency demands respect, necessitating that users follow safety and application guidelines diligently.
Oxalic Acid Structure
What is Oxalic Acid?
Oxalic acid is a simple dicarboxylic acid with the chemical formula C2H2O4. It's a crystalline solid that appears as a white substance at room temperature. In various industries, oxalic acid is used for cleaning, rust removal, and other applications.
Structural Details
Oxalic acid consists of two carboxylic acid groups (-COOH) connected directly to each other. Its structure can be visualized as:
HOOC-COOH
The molecule is planar, and due to the presence of two carboxylic acid groups, it can form strong hydrogen bonds with other molecules, leading to its relatively high melting point for a molecule of its size.
Properties Influenced by its Structure
- Solubility: Due to its ability to form hydrogen bonds, oxalic acid is soluble in water.
- Acidity: Being a dicarboxylic acid, oxalic acid can donate two protons, making it a relatively strong acid.
- Reactivity: The acid groups in the molecule allow it to participate in various chemical reactions, such as complexation with metal ions.
Industrial Uses
Oxalic acid finds applications in various industries. Some common uses include:
- Cleaning agents, especially for the removal of rust.
- In the textile industry for bleaching and dyeing processes.
- As a mordant in dyeing processes.
- For the extraction of rare earth metals.
Precautions
While oxalic acid has many applications, it is toxic and can be corrosive. Direct exposure should be avoided, and appropriate safety measures, such as gloves and goggles, should be used when handling this chemical.
Oxalic Acid Formula
Oxalic acid is a simple dicarboxylic acid with a notable presence in various industries due to its potent properties. Understanding its chemical formula is fundamental to grasping its nature and applications.
Chemical Formula of Oxalic Acid
The chemical formula for oxalic acid is C2H2O4.
Structural Aspects
Oxalic acid consists of two carboxylic acid groups (-COOH) linked together. The presence of these acidic groups is what gives oxalic acid its characteristic properties. The structural formula can be represented as HOOC-COOH.
Properties of Oxalic Acid
Some of the noteworthy properties of oxalic acid include:
- Physical State: In its anhydrous form, it is a white crystalline solid.
- Solubility: Oxalic acid is soluble in water, alcohol, and ether.
- Acidic Nature: Being a dicarboxylic acid, it can donate two protons, making it a strong acid compared to many organic acids.
Applications of Oxalic Acid
Beyond its chemical specifications, oxalic acid has various practical applications.
- Cleaning Agent: It's often used as a rust remover and general cleaning agent in many settings.
- Bleaching: Oxalic acid can act as a bleaching agent, especially in wood processing.
- Industrial Uses: It serves as a mordant in dyeing processes and plays roles in several chemical syntheses.
Oxalic acid, represented by the formula C2H2O4, is a versatile compound with a wide range of uses across various industries. Proper knowledge and safe handling are imperative when working with this acid.
Oxalic Acid Dihydrate
What is Oxalic Acid Dihydrate?
Oxalic acid dihydrate is the hydrated form of oxalic acid. Its chemical formula is C2H2O4·2H2O. It appears as a white, crystalline solid at room temperature. When heated, the dihydrate loses its water molecules, reverting back to anhydrous oxalic acid.
Structural Details
In the dihydrate form, oxalic acid has two water molecules associated with each molecule of the acid. These water molecules are weakly bound and can be easily removed upon heating.
Physical Properties
- Molecular Weight: 126.07 g/mol (due to the additional weight of the two water molecules).
- Appearance: White crystalline solid.
- Solubility: Soluble in water due to its ability to form hydrogen bonds.
- Melting Point: Begins to lose its water molecules around 101.5°C.
Industrial Uses
Oxalic acid dihydrate has various applications, including:
- As a reducing agent in photography.
- In the preparation of certain pharmaceuticals.
- For cleaning and bleaching, especially in the removal of rust.
- In laboratories as a standard for calibrating certain types of equipment.
Precautions
Oxalic acid dihydrate, like its anhydrous counterpart, is toxic and can be corrosive. It's essential to handle this compound with care, avoiding direct skin contact and inhalation. When working with it, the use of gloves, goggles, and other safety equipment is recommended.
Storage
It should be stored in a cool, dry place away from incompatible substances like strong bases or reducing agents. Its container should be tightly sealed to prevent moisture absorption or contamination.
Who is Soapgoods?
We are proud to present a diverse and extensive selection of soap making supplies including soap molds as well as melt and pour soap bases. Are you looking for something unique, something hard to find? Wondering Where to buy Witch Hazel Distillate - Alcohol Free? We are a fantastic source!
We carry it all and many other fantastic but hard to find items at great wholesale prices, we are the one-stop-shop, you can find everything you need here, even bulk Xanthan Gum Right here at Soapgoods
We know you have choices, that's why we work harder, providing better quality, faster shipping and a wide selection of items in practical sizes for any application. When you need to make a purchase and need it delivered quickly, look to us to provide you the service you need. We even have Wholesale Yogurt Powder, in small and large builds sizes, either way, you can get our best direct to consumer pricing. Plus we ship it out the next business days. and will ship the next business day.
Where else can you do all your shopping in one place? When you need to make a purchase and get it delivered quickly. Buy Zinc Oxide Powder? Right here
When you are looking for quality personal care products, cosmetics and soap making supplies, be sure to visit our online store for Acacia Gum Enjoy our premium quality ingredients and super fast shipping times.
Read our Real Reviews from Google and Bizrate
100% Real Reviews (we cannot edit, create or delete reviews) From Google, Click here -> Real SoapGoods Reviews
See more Real Reviews (we cannot edit, create or delete reviews) from Third Party Watchdog Bizrate Real SoapGoods Reviews (Click Here and Scroll Down)
Read hundreds of real testimonials from over a decade of service. SoapGoods Testimonials.
How Fast Can I get it?
We Guarantee Your order ships out the same or next business day! This means in the South East you will have your order in 1 to 3 business days, in the North East normally 3 to 4 days and in the West normally 4 to 5 days. For full details on shipping and processing times please see our expected delivery times.
These times are based on business days, not including weekends or holidays.
FedEx Delivery Map
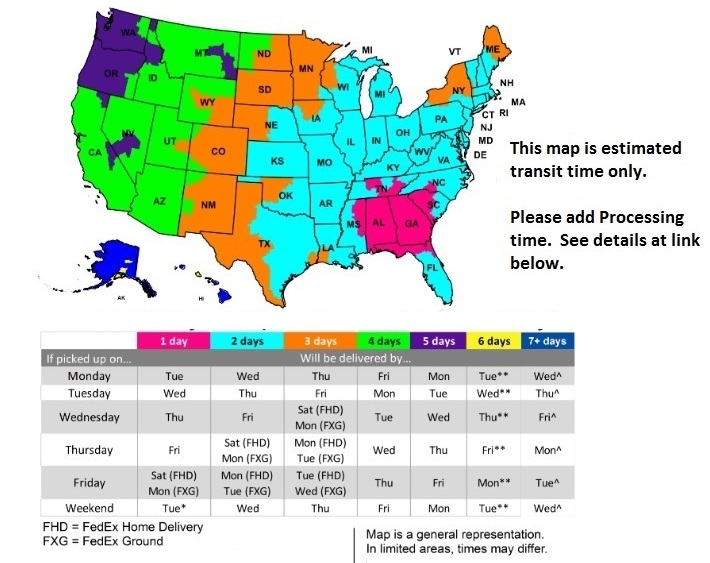
For Processing Times Click Here
USPS Delivery Map
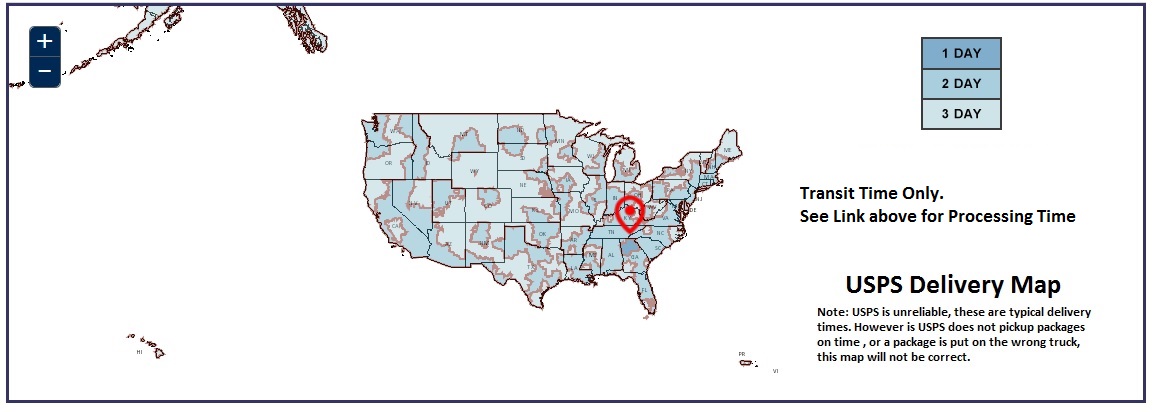
Typical Delivery Times to Major US Cities
| Major Cities | Total Business Days +1 / -1 |
|---|---|
| Alabama (AL) - Montgomery, Birmingham | 1 |
| Alaska (AK) - Juneau, Anchorage | 7 |
| Arizona (AZ) - Phoenix, Tucson | 4 |
| Arkansas (AR) - Little Rock, Fayetteville | 2 |
| California (CA) - Sacramento, Los Angeles, San Francisco, San Diego, Sacramento, San Jose | 4 |
| Colorado (CO) - Denver, Colorado Springs | 3 |
| Connecticut (CT) - Hartford, New Haven | 2 |
| Delaware (DE) - Dover, Wilmington, Newark | 2 |
| Florida (FL) - Tallahassee, Orlando, Miami, Jacksonville, Tampa, Destin | 2 |
| Georgia (GA) - Atlanta, Savannah, Augusta, Athens | 1 |
| Hawaii (HI) - Honolulu, Kailua | 7 |
| Idaho (ID) - Boise, Coeur d'Alene | 4 |
| Illinois (IL) - Springfield, Chicago, Peoria, Rockford | 2 |
| Indiana (IN) - Indianapolis, Fort Wayne | 2 |
| Iowa (IA) - Des Moines, Cedar Rapids | 2 |
| Kansas (KS) - Topeka, Wichita, Kansas City | 2 |
| Kentucky (KY) - Frankfort, Louisville, Lexington | 2 |
| Louisiana (LA) - Baton Rouge, New Orleans, Lafayette | 2 |
| Maine (ME) - Augusta, Portland, Bangor | 3 |
| Maryland (MD) - Annapolis, Baltimore | 2 |
| Massachusetts (MA) - Boston, Cambridge, Worcester | 2 |
| Michigan (MI) - Lansing, Detroit, Grand Rapids | 2 |
| Minnesota (MN) - St. Paul, Minneapolis, Duluth | 3 |
| Mississippi (MS) - Jackson, Biloxi, Hattiesburg | 1 |
| Missouri (MO) - Jefferson City, St Louis, Kansas City | 2 |
| Montana (MT) - Helena, Billings | 4 |
| Nebraska (NE) - Lincoln, Omaha | 2 |
| Nevada (NV) - Carson City, Las Vegas, Reno | 4 |
| New Hampshire (NH) - Concord, Manchester, Portsmouth | 2 |
| New Jersey (NJ) - Trenton, Newark, Jersey City | 2 |
| New Mexico (NM) - Santa Fe, Alburquerque | 3 |
| New York (NY) - Albany, New York, Rochester, Buffalo, Albany, Syracuse, Niagara Falls, Ithaca | 3 |
| North Carolina (NC) - Raleigh, Charlotte | 2 |
| North Dakota (ND) - Bismarck, Fargo | 3 |
| Ohio (OH) - Columbus, Cleveland, Cincinnati | 2 |
| Oklahoma (OK) - Oklahoma City, Fairview, | 2 |
| Oregon (OR) - Salem, Portland, Eugene | 5 |
| Pennsylvania (PA) - Harrisburg, Philadelphia, Pittsburgh | 2 |
| Rhode Island (RI) - Providence, Newport | 2 |
| South Carolina (SC) - Columbia, Charleston | 1 |
| South Dakota (SD) - Pierre, Sioux Falls, Rapid City | 3 |
| Tennessee (TN) - Nashville, Memphis | 2 |
| Texas (TX) - Austin, Houston, Dallas | 3 |
| Utah (UT) - Salt Lake City, St. George | 3 |
| Vermont (VT) - Montpelier, Burlington | 3 |
| Virginia (VA) - Richmond, Virginia Beach | 2 |
| Washington (WA) - Olympia, Seattle, Vancouver, Spokane | 5 |
| West Virginia (WV) - Charleston, Morgantown | 2 |
| Wisconsin (WI) - Madison, Milwaukee | 2 |
| Wyoming (WY) - Cheyenne, Jackson | 4 |
Disclaimer: All product descriptions and specifications provided in this description are intended as a guide only and are subject to change without notice. While we strive for accuracy, discrepancies or errors may be present. These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
No posts found











